CRISTAVISC® C

VISCOELASTIC COHESIVE
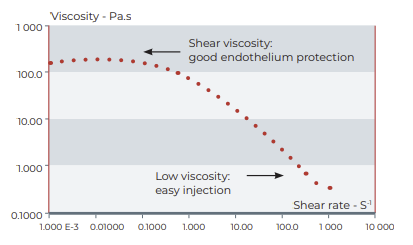
With an average 170 000 millipascal second zero shear viscosity, CRISTAVISC® c is ideally cohesive.
| DESIGNATION | TECHNICAL SPECIFICATIONS |
|---|---|
| Eur. Ph. Injectable quality hyaluronic acid for Intraocular use, from biofermentation origin | — |
| Each box contains | 1 syringe – 1 x 27G 7/8” canula – 1 leaflet – 8 traceability labels |
| Glass syringe, Pharmaceutical grade, Class I, latex free, prefilled | At 1 ml |
| NaHa concentration | 15,5 mg/g |
| Molecular weight of the hyaluronic acid in the final sterile product | 2.2 MDa (mean value) |
| Phosphate Buffer pH 7,2 | q.s. 1g |
| With natural antioxidant | Hyaluronic acid with mannitol |
| Isoosmolarity | 310 most (mean value) |
| pH | 6.8 – 7.6 |
| Viscosity at 0,01s-1 shear rate | 170 Pa.s (170 000 cPoise) (mean value) |
| Apyrogen (free of endotoxins) | < 0.5 EU/g |
| Sterile (gel by autoclave – second packaging, blister by ETO) | SAL 10-6 (Sterility Assurance Level) |
| Proteins | < 20 ppm |
| Storage | 2°C to 25°C for up to 36 months |
| Medical Device | Class IIb |
| Biocompatible according to | ISO 10993 and ISO 15798 |
| System of quality management in conformity to | ISO 13485 |
Request for more information
Feel free to request for a call back if you are interested in any of our listed products. Our friendly advisers are more than happy to answer any queries that you may have!
